HISTORY
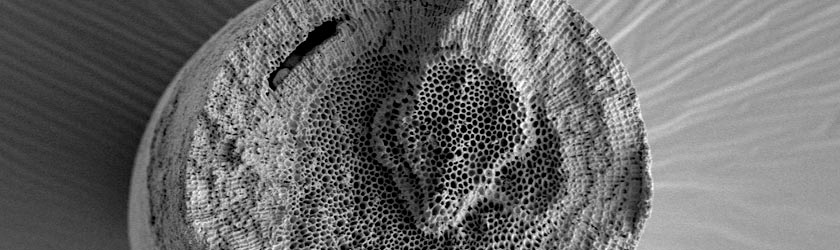
Since the year 1982, we occupy ourselves with bone-forming materials in the area of the bionics. The basic idea was to produce synthetically an interconnecting microporous material. But in scientific examinations it has been found out that the mineral hard tissue scaffold (skeleton) of some naturally grown red algal species represents an ideal basic structure to produce a biomimetic product with interconnecting pores.It had been established that the carbonate-skeleton of these red-algae can be transformed by hydrothermal processes into hydroxyapatite as well as tricalciumphosphate. The chemical composition of the final product is very similar to the natural bone and its morphology resembles the human dentin.

Comparison of structure of human dentin (left) and Corallina officinalis (right).
The first animal experiments were very promising. These primary studies proved the very good osteoconductivity of the red algae derived material. However, because of the comparatively short observation period, a very important quality the resorbability of this material could not be detected.
Only in animal experiments with longer observation periods as well as later in the clinical application it was established that the material shows excellent resorption-kinetics. By that means, reticular, newly formed bone structures in the sense of the trabecular structure will be built early after surgery which enables an excellent remodelling.
Fortunately, the scientific projects were supported by DFG (Deutsche Forschungsgemeinschaft) as well as the scientific foundation of Schleswig-Holstein, so that 1987 after patent applications the first delivery-contract with the company then called "Friedrichsfeld" (today "Dentsply Sirona Implants") could be signed.
1998 the pure phycogenic (algae derived) hydroxyapatite material has been CE- certified under the brand Algipore® by Friatec GmbH (today Dentsply Sirona Implants).
AlgOss Biotechnologies received approval for the European market for its own AlgOss products in 2010.
In 2014, the phycogenic product range was expanded to include Symbios® Biphasic Bone Graft Material (HA/TCP: 20-80) and, in 2018, Symbios® Algipore®.
The choice of different mono and biphasic material variants allows the user to achieve the best possible bone regeneration.
Moreover the specific, unique morphology of the phycogenic granulates has a great potential as a carrier for bone-inductive substances.